Periodic Classification of Elements Questions
Multiple Choice Questions
1. Upto which element, the Law of Octaves was found to be applicable
(a) Oxygen
(b) Calcium
(c) Cobalt
(d) Potassium
Ans: (b)
2. According to Mendeleev's Periodic Law, the elements were arranged in the periodic table in the order of
(a) increasing atomic number
(b) decreasing atomic number
(c) increasing atomic masses
(d) decreasing atomic masses
Ans: (c)
3. In Mendeleev's Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later
(a) Germanium
(b) Chlorine
(c) Oxygen
(d) Silicon
Ans: (a)
General Questions
1. Can the following groups of elements be classified as Dobereiner's triad?
(a) Na, Si, Cl
(b) Be, Mg, Ca
Atomic mass of Be 9; Na 23; Mg 24; Si 28; Cl 35; Ca 40
Explain by giving reason.
2. Mendeleev's Periodic Table the elements were arranged in the increasing order of their atomic masses. However, cobalt with an atomic mass of 58.93 amu was placed before nickel having an atomic mass of 58.71 amu. Give reason for the same.
3. Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev.
4. Mendeleev predicted the existence of certain elements not known at that time and named two of them as Eka-silicon and Eka-aluminium.
(a) Name the elements which have taken the place of these elements
(b) Mention the group and the period of these elements in the Modern Periodic Table.
(c) Classify these elements as metals, non-metals or metalloids
(d) How many valence electrons are present in each one of them?
5. Which group of elements could be placed in Mendeleev's Periodic Table without disturbing the original order? Give reason.
6. Give an account of the process adopted by Mendeleev for the classification of elements. How did he arrive at “Periodic Law”?
7. How were the positions of cobalt and nickel resolved in the Modern Periodic Table?
Ans:
The atomic number of cobalt is 27 and that of nickel is 28. Now according to modern periodic law, the elements are arranged in the order of increasing atomic numbers. So, the cobalt with a lower atomic number should come first and nickel with a higher atomic number should come later.
8. Consider the isotopes of chlorine, Cl-35 and Cl-37. Would you place them in different slots because their atomic masses are different? Or would you place them in the same position because their chemical properties are the same?
Ans:
In the same slot. Reason, they have the same chemical properties (depends on the atomic number) .
9. How were the positions of isotopes of various elements decided in the Modern Periodic Table?
Ans:
Since all the isotopes of an element have the same atomic number, they can be put in one place in the same group of the periodic table. For example, both the isotopes of chlorine, Cl-35 and Cl-37 have the same atomic number of 17. So, both of them can be put in one place in the same group at the periodic table.
10. The three elements A, B and C with similar properties have atomic masses X, Y and Z respectively. The mass of Y is approximately equal to the average mass of X and Z. What is such an arrangement of elements called as? Give one example of such a set of elements
11. Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the Modern Periodic Table. Also, give their valencies.
Ans:
A 👉 Group 13
B 👉 Group 14
C 👉 Group 2
Valencies
A 👉 3
B 👉 4
C 👉 2
12. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
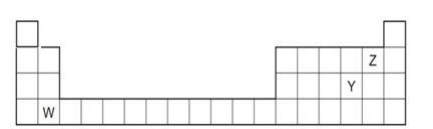
13. The diagram below shows part of the periodic table.
a. Which elements would react together to form covalent compounds?
b. Between the two elements W and Z, which will have a bigger atomic
radius? Why?
14. Compare the radii of two species X and Y. Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
Ans:
Both X and Y have 12 protons therefore the atomic number is 12, i.e, magnesium. Y have only 10 electrons, the effect of the nuclear charge is greater in Y as compared to X. Therefore radius of Y is smaller as compared to X.
15. In the following diagram for the first three periods of the periodic table, five elements have been represented by the letters `a, b, c, d` and `e`
(ii) Select the letter which represents a noble gas.
(iii) What type of bond is formed between `a` and `b?`
(iv) What type of bond is formed between `c` and `b?`
(v) Which element will form a divalent anion?
16. Given alongside is a part of the periodic table:
As we move horizontally from left to right:
(i) What happens to the metallic character of the element?
(ii) What happens to the atomic size?
17. The following diagram shows a part of the periodic table in which the elements are arranged according to their atomic number.
(ii) which element has a higher valency, `k or o?`
(iii) which element is more metallic, `i or k`?
(iv) which element is more non-metallic `d or g?`
(v) select a letter that represents a metal of valency 2.
(vi) select a letter that represents non-metals of valency 2.
18. The atomic numbers of three elements A, B and C are given below:
(i) which element belongs to group 18?
(ii) which element belongs to group 15?
(iii) which element belongs to group 13?
19. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide
20. The following shows the position of five elements A, B, C, D, E in the modern periodic table.
i) which element is a metal with valency two?
ii) which element is least reactive?
iii) Out of D and E which element has a smaller atomic radius?





Useful questions 🤘🤘
ReplyDeleteThank you
DeleteGood ques.🤘
ReplyDeleteThank you
Delete
ReplyDeleteImportant questions
Thank you
Delete